In patients with non-small cell lung cancer (NSCLC) harbouring EGFR mutations, patients with an exon 21 L858R mutation generally face a poorer progression-free survival (PFS) as compared to patients with exon 19 deletions. Recent results from the RELAY study, presented by Nagakawa et al., now indicate that the combination of ramucirumab and erlotinib overcomes this dismal prognosis by yielding a similar PFS objective response rate, duration of response and time to second disease progression in metastatic NSCLC (mNSCLC) patients with an exon 21 L858R mutation as in patients harbouring an exon 19 deletion (ex19el). In addition, the presented analysis indicated that also the need for chemotherapy was delayed with ramucirumab plus erlotinib compared to erlotinib alone in mNSCLC patients with an exon 21 L858R mutation.
INTRODUCTION
EGFR mutations represent the most frequent oncogeneic driver mutation in patients with NSCLC and can be found in 40-60% of Asian patients and 10-20% of Caucasian patients. The most common of these genetic aberrations consist of ex19del and exon 21 L858R mutations, making up approximately 90% of all EGFR mutations. The presence of these activating EGFR mutations in advanced NSCLC is associated with sensitivity to small-molecule EGFR tyrosine kinase inhibitors (TKIs), which paved the way for a molecularly targeted treatment approach in this setting. Although EGFR targeting TKIs outperform chemotherapy in all EGFR mutant mNSCLC patients, the degree of benefit differs between mutation types, with a more pronounced benefit in patients with an ex19del than in patients with an exon 21 mutation. In fact, subgroup analyses of the different landmark trials with first-, second- and third-generation EGFR TKIs all indicate that the extent of progression-free survival (PFS) benefit is less pronounced in patients with exon 21 L858R mutations than in patients with an ex19del. To date, the longest median PFS obtained with a single agent anti-EGFR TKI in patients with an exon 21 L858R mutation was reported at 14.4 months. This finding was further confirmed by a meta-analysis presented at ESMO 2020.1 Recently, the combination of EGFR-TKIs with antiangiogenic agents has emerged as a strategy to delay the development of resistance and prolong disease control through dual inhibition of VEGF and EGFR pathways.2
In the phase III RELAY trial, 449 patients with previously untreated mNSCLC harbouring an EGFR ex19del or exon 21 L858R mutation were randomised (1:1) to receive erlotinib (150 mg/day) with either ramucirumab (10 mg/kg) or placebo once every two weeks, until RECIST1.1-defined progression or unacceptable toxicity. Patients with CNS metastases at baseline were excluded from the trial. Overall, baseline characteristics were well balanced across treatment groups and across EGFR mutation types. However, exon 21 L858R mutations did prove to be more prevalent in older patients, in patients of Asian origin and in females. In addition, bone metastases were also more frequently seen in patients with exon 21 L858R mutation. Overall, the RELAY trial demonstrated that ramucirumab plus erlotinib significantly prolongs the PFS compared to erlotinib plus placebo, with a median PFS of 19.4 and 12.4 months, respectively (HR[95%CI]: 0.59[0.46-0.76], p< 0.0001). Importantly, this PFS benefit in favour of the ramucirumab-erlotinib combination was consistently seen in all prespecified subgroups, irrespective of the EGFR mutation type (ex19del stratified log-rank p= 0.0032; exon 21 L858R mutation stratified log-rank p= 0.0038).2 During ESMO 2020, Nagakawa et al. et al reported the results of a more in-depth analysis of the RELAY trial in function of the EGFR mutation status, with a particular focus on the secondary endpoints of the trial.3
RESULTS
As reported earlier, the addition of ramucirumab to erlotinib significantly prolonged the PFS, irrespective of the mutation type. In patients with an ex19del, the median PFS was prolonged from 12.5 to 19.6 months (HR[95%CI]: 0.595[0.420-0.842]; p= 0032), corresponding to a 1-year PFS rate of 74% and 54% for ramucirumab-erlotinib and placebo-erlotinib, respectively. In the exon21 L858R cohort, similar findings were reported with a median PFS of 19.4 vs. 11.2 months (HR[95%CI]: 0.588[0.409-0.846]; p= 0.0038) and a 1-year PFS rate of 70% vs. 47% for ramucirumab-erlotinib and placebo-erlotinib, respectively.
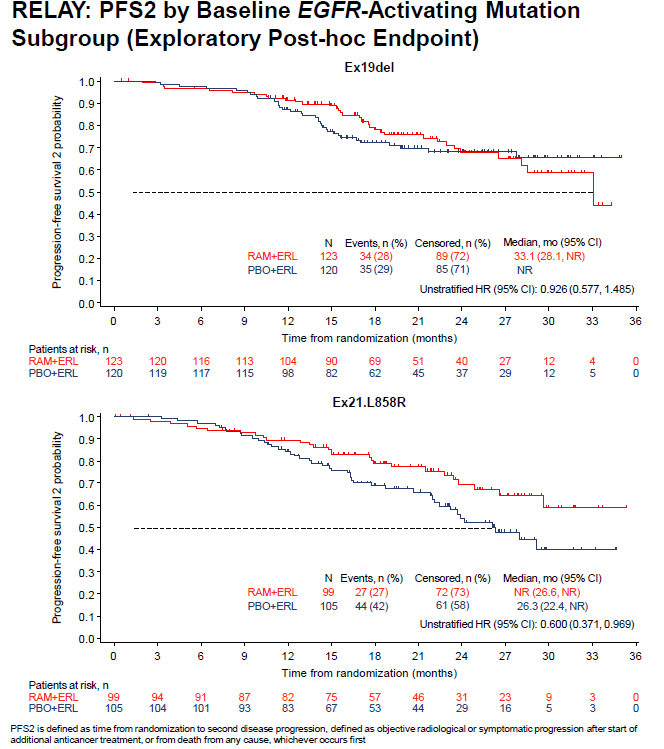
The data on the time to the second disease progression (PFS2) data were still immature at the time of the analysis (overall censoring rate = 68%), with the lowest censoring rate observed for the placebo plus erlotinib in the exon 21 L858R subgroup. This indicates that more PFS2 events had occurred in this cohort compared to the other mutation subgroups. Exon 21 L858R patients treated with ramucirumab plus erlotinib continued to exhibit an improved benefit through PFS2 (HR[95%CI]: 0.600[0.371-0.969]), representing a 40% reduction in the risk of second disease progression or death upon the addition of ramucirumab. In contrast, for patients with an ex19del, no difference in PFS2 was observed between both treatment arms (HR[95%CI]: 0.926[0.577-1.485]). Also with respect to the time-to-chemotherapy (TTCT) ramucirumab plus erlotinib outperformed placebo plus erlotinib. Interestingly, the presented analysis in function of EGFR mutation type confirmed this finding in the exon 21 L585R subgroup (HR[95%CI]: 0.554[0.352-0.872]; censoring rate: 61%), while this was not the case in the subgroup of patients with an ex19del (HR[95%CI]: 1.034[0.682-1.567]; censoring rate: 63%). Of note, given the fact that exon 19 deletions are more common in Western patients, who were underrepresented in RELAY, these results are to be interpreted with caution (i.e. only 28% of ex19del patients were white/Caucasian).
The ORR with ramucirumab-erlotinib was fairly similar in patients with an ex19del (79%) or an exon 21 L858R mutation (74%). This was not the case for placebo-erlotinib where an ORR of 83% was seen in ex19del patients vs. 66% in the exon 21 L858R cohort. Irrespective of the EGFR mutation, the duration of response (DoR) favoured the ramucirumab-erlotinib combination over placebo-erlotinib (median DoR: ex19del 18.2 vs. 11.0 months, HR[95%CI]: 0.542[0.380-0.772]; exon 21 L858R 16.2 vs. 1.1 months; HR[95%CI]: 0.731[0.493-1.083]).3
Finally, the safety profiles were as expected and did not differ between the two EGFR mutations subtype.3
CONCLUSION
Metastatic NSCLC patients harbouring an exon 21 L858R mutation experienced a similar clinical benefit (PFS, ORR, DoR, PFS2) with ramucirumab-erlotinib as patients with an ex19del. As such, the combination of ramucirumab and erlotinib seems to overcome the known dismal prognostic consequences of having an exon 21 EGFR mutation. Interestingly, despite high censoring, the use of chemotherapy was delayed with ramucirumab plus erlotinib versus placebo and erlotinib in exon 21 L858R patients. Overall, the efficacy and safety results tin this analysis support the combination of ramucirumab and erlotinib as a first-line therapy for patients with metastatic NSCLC, irrespective of the EGFR mutation type.
References