BJMO - volume 17, issue 4, june 2023
P. Giron PhD, J. De Grève MD, PhD
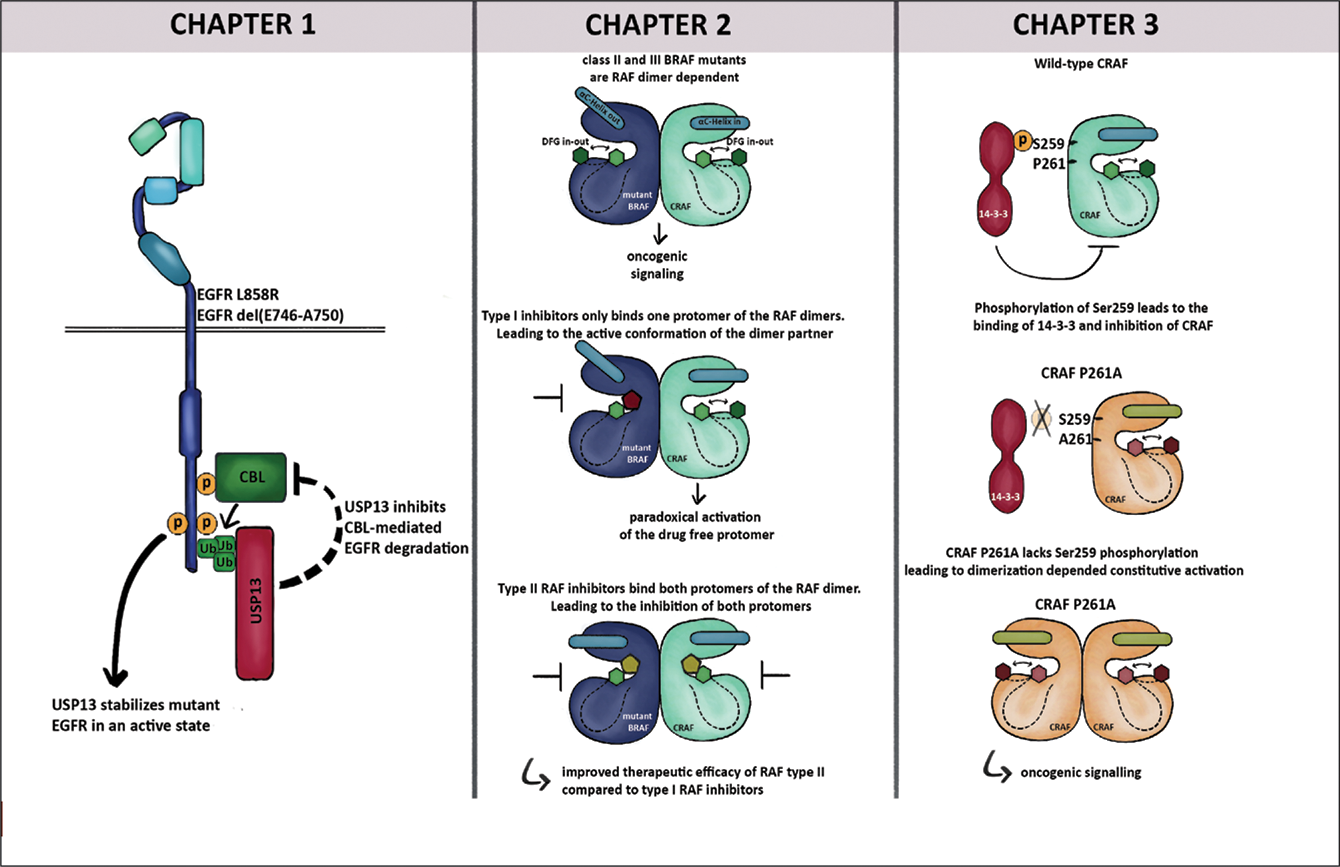
GRAPHICAL ABSTRACT
(Belg J Med Oncol 2023;17(4):132–4)
Read more
BJMO - volume 15, issue 6, october 2021
I. Vergote MD, PhD, H. Denys MD, PhD, J. De Grève MD, PhD, C. Gennigens MD, PhD, K. Van de Vijver MD, PhD, J. Kerger MD, P. Vuylsteke MD, J-F. Baurain MD, PhD
Ovarian cancer is often diagnosed at an advanced stage, which is associated with worse survival outcomes and more limited therapeutic options. Over the last years, knowledge regarding the molecular features of ovarian cancer has advanced considerably, enabling the development of several options for diagnosis and treatment in a patient-tailored approach. Identification of homologous recombination deficiency (such as mutations of the BRCA1 and BRCA2 genes, or genomic instability) affecting DNA repair, has become essential in guiding treatment decisions, especially after the development of targeted agents. Therapeutic decisions take into consideration the cancer subtype, its molecular features and disease stage. Fundamental principles of good treatment for women with ovarian cancer include debulking surgery (to reduce the tumour to no residual disease whenever possible), along with appropriate systemic treatment (chemotherapy and targeted agents). To aid Belgian physicians in developing the best individual medical strategies for patients with primary and recurrent ovarian cancer, we present here standard of care applicable in Belgium, that also includes recently developed targeted agents and currently applicable reimbursement criteria.
(BELG J MED ONCOL 2021;15(6):286-91)
Read moreBJMO - volume 14, issue 3, may 2020
L. Decoster MD, PhD, C. Kenis RN, PhD, H. Wildiers MD, PhD, J. De Grève MD, PhD
As the cancer population ages, treatment decisions in the older patients should not only be guided by the tumour characteristics but also by patient characteristics. The performance of a comprehensive geriatric assessment as well as a health related quality of life evaluation are important in order to deliver the optimal personalised care in older patients with cancer. The current PhD thesis focused on the use of screening tools, geriatric assessment and interventions as well as on health-related quality of life in older patients with cancer.
(BELG J MED ONCOL 2020;14(3):106–8)
Read moreBJMO - volume 14, issue 3, may 2020
J. De Grève MD, PhD, M. Peeters MD, PhD, V. Remouchamps MD, PhD, Y. Lievens MD, PhD, M. Lambrecht MD, PhD
These guidelines are a summary derived from national and international guidelines.1–7
Read moreBJMO - volume 13, issue 7, november 2019
Ir A. Hébrant PhD, M. Lammens MD, PhD, C. Van den Broecke MD, N. D’Haene MD, PhD, J. Van den Oord MD, PhD, A. Vanderstichele MD, PhD, A. Dendooven MD, PhD, P. Neven MD, PhD, K. Punie MD, G. Floris MD, PhD, J. Van der Meulen MD, HA. Poirel MD, PhD, C. Dooms MD, PhD, S. Rottey MD, PhD, T. Boterberg MD, PhD, L. Brochez MD, PhD, M.C. Burlacu MD, G. Costante MD, D. Creytens MD, PhD, P. De Paepe MD, PhD, R. De Pauwn MD, B. Decallonne MD, PhD, F. Dedeurwaerdere MD, H. Denys MD, PhD, L. Ferdinande MD, PhD, R. Forsyth MD, PhD, M. Garmyn MD, PhD, T. Gevaert MD, PhD, J. De Grève MD, PhD, E. Govaerts MD, E. Hauben MD, PhD, J. Kerger MD, O. Kholmanskikh Van Criekingen MD, PhD, V. Kruse MD, PhD, Y. Lalami MD, L. Lapeire MD, PhD, P. Lefesvre MD, PhD, J.P. Machiels MD, PhD, B. Maes MD, PhD, G. Martens MD, PhD, M. Remmelink MD, PhD, I. Salmon MD, PhD, R. Sciot MD, PhD, S. Tejpar MD, PhD, K. Van de Vijver MD, PhD, L. Van de Voorde MD, I. Van den Berghe MD, A. Van den Bruel MD, K. Vandecasteele MD, PhD, L. Vanwalleghem MD, K. Vermaelen MD, PhD, R. Salgado MD, PhD, E. Wauters MD, PhD, B. Weynand MD, PhD, E. Van Valckenborgh PhD, G. Raicevic PhD, M. Van den Bulcke PhD, P. Pauwels MD, PhD
In order to advise the Federal Government on the reimbursement of molecular tests related to Personalised Medicine in Oncology, the Commission of Personalised Medicine (ComPerMed), represented by Belgian experts, has developed a methodology to classify molecular testing in oncology. The different molecular tests per cancer type are represented in algorithms and are annotated with a test level reflecting their relevance based on current guidelines, drug approvals and clinical data. The molecular tests are documented with recent literature, guidelines and a brief technical description. This methodology was applied on different solid tumours for which molecular testing is a clear clinical need.
(BELG J MED ONCOL 2019;13(7):286–95)
Read moreBJMO - volume 13, issue 2, march 2019
Ir A. Hébrant PhD, K. Punie MD, F.P. Duhoux MD, PhD, C. Colpaert MD, PhD, G. Floris MD, PhD, K. Lambein MD, PhD, P. Neven MD, PhD, M. Berlière MD, PhD, R. Salgado MD, PhD, M. Chintinne MD, PhD, K. Dahan MD, PhD, S. Dedeurwaerdere MD, J. De Grève MD, PhD, A. de Leener MD, PhD, H. Denys MD, PhD, R. de Putter MD, L. Desmyter PhD, M. Baldewijns MD, PhD, D. Feret MD, C. Fontaine MD, C. Galant MD, P. Hilbert PhD, J. Janssens MD, PhD, D. Larsimont MD, PhD, P. Lefesvre MD, PhD, T. Sticca PhD, M-D. Tkint de Roodenbeke MD, G. Van Den Eynden MD, PhD, I. Vanden Bempt MD, PhD, C. Van den Broecke MD, I. Vandernoot MD, C. Sotiriou MD, PhD, J. van Dorpe MD, PhD, H.A. Poirel MD, PhD, E. Van Valckenborgh PhD, G. Raicevic PhD, M. Van den Bulcke PhD, P. Aftimos MD
In order to advise the Federal Government on all matters related to personalised medicine in oncology, including the reimbursement of molecular tests, the Commission of Personalized Medicine (ComPerMed) has applied, for the breast tumours, the same methodology as previously applied for the digestive tumours. Meaning, the different molecular tests, represented in the shape of algorithms, are annotated with test levels — which aim to reflect their relevance based on current available data and to define the reimbursement — and are documented with recent literature, guidelines and a brief technical description.
(BELG J MED ONCOL 2019;13(2):40–45)
Read moreBJMO - volume 13, issue 9, february 2019
Tom Feys MBA, MSc, J. De Grève MD, PhD
Chimeric antigen receptor (CAR) T-cell therapy is a new cancer immunotherapy targeting specific cell surface antigens. This type of adoptive cell immunotherapy has been a breakthrough in the treatment of aggressive B-cell lymphoma and B-cell precursor acute lymphoblastic leukaemia (ALL) and is currently being studied in other cancer types, including multiple myeloma and chronic lymphocytic leukaemia. With the unprecedented success of CAR T cells in haematological malignancies, a growing number of (pre)clinical studies are focusing on translating this treatment to solid tumours. However, response rates to CAR T-cell therapy have so far been much less favourable in non-haematologic malignancies, mainly due to a paucity of unique tumour target antigens, limited CAR T-cell trafficking to tumour sites, tumour heterogeneity, antigen loss, the presence of an immune suppressive tumour microenvironment and recognition of normal cells expressing the targeted antigen. A broad range of strategies is currently being explored to overcome these hurdles. For example, TCR-CAR-T hybrids have been developed that can also target intracellular antigens which broadens the potential scope of the CAR-T cell strategy. This article reviews completed and ongoing CAR T-cell trials in solid tumours and discusses the strategies to improve the efficacy of this treatment modality in solid tumours, including accelerated production flows. CAR-T’s might very well be the upcoming major advance in cancer treatment.
Read moreTo provide the best experiences, we and our partners use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us and our partners to process personal data such as browsing behavior or unique IDs on this site and show (non-) personalized ads. Not consenting or withdrawing consent, may adversely affect certain features and functions.
Click below to consent to the above or make granular choices. Your choices will be applied to this site only. You can change your settings at any time, including withdrawing your consent, by using the toggles on the Cookie Policy, or by clicking on the manage consent button at the bottom of the screen.